Philip Conaghan, MBBS, PhD, FRACP, FRCP
Professor of Musculoskeletal Medicine; Director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine

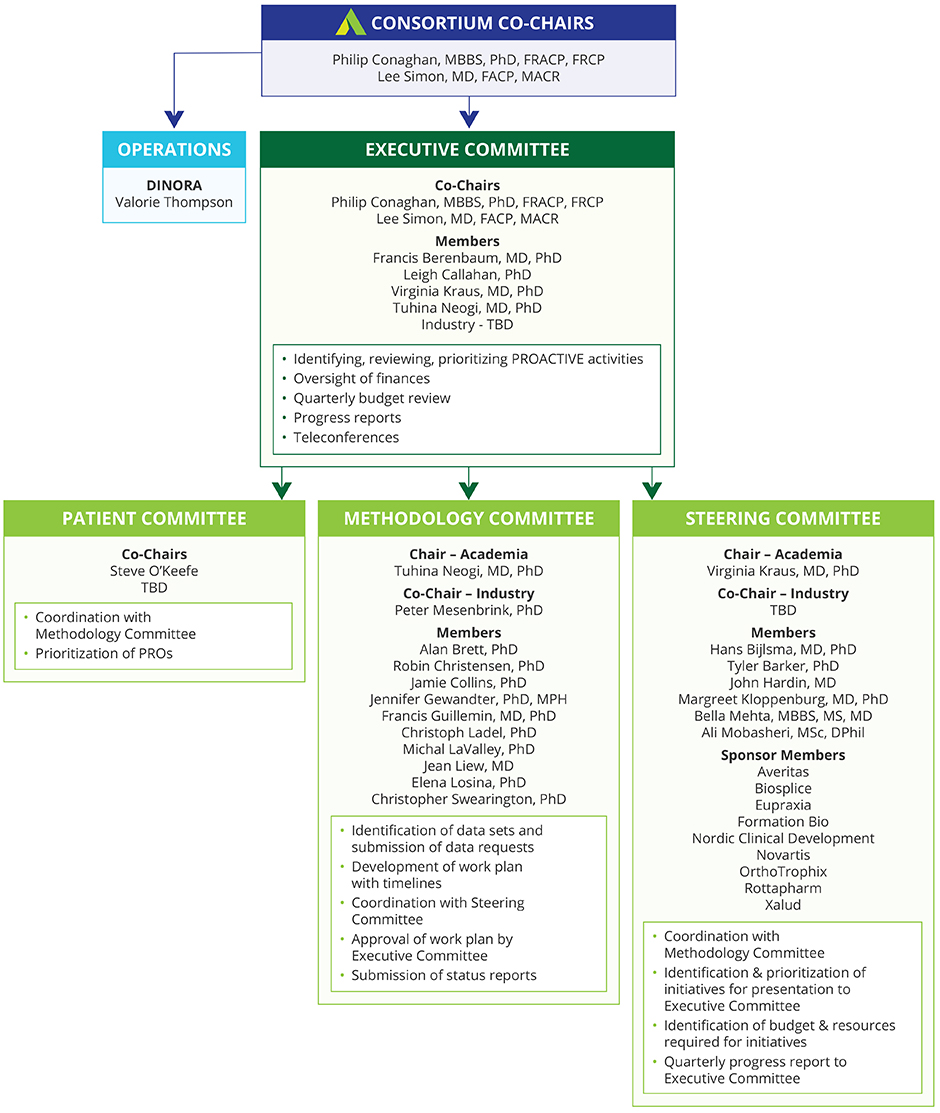
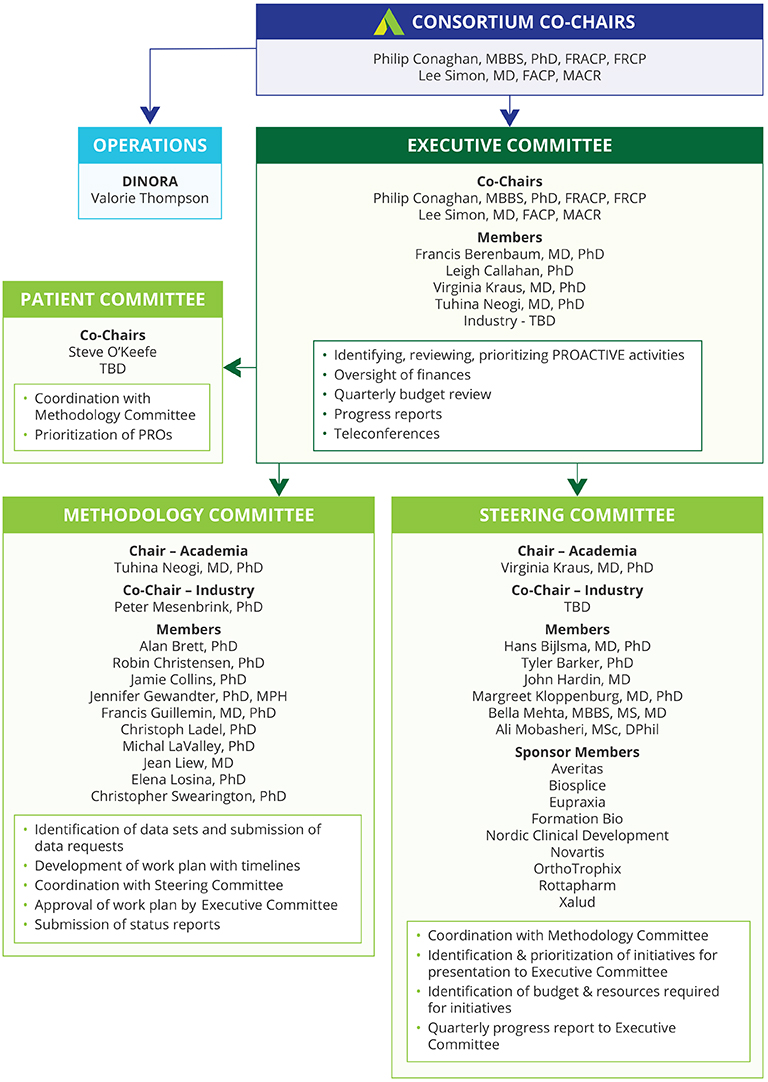
Initiating and supporting a public private, pre-competitive collaboration among a broad spectrum of stakeholders, including academia, government agencies, industry, professional organizations, and patient organizations with the goal of sharing data and innovative thinking about the development of OA outcome measures for clinical trials of therapies aimed at slowing OA progression.
The Food and Drug Administration approved PROACTIVE’s application seeking participation by individuals from the Center for Drug Evaluation and Research (CDER) to engage in the activities of the consortium.
There are more than 100 types of arthritis with osteoarthritis (OA) being the most prevalent. Worldwide, 242 million people have symptomatic and activity-limiting OA of the hip and/or knee. It is the third most rapidly rising condition associated with disability, just behind diabetes and dementia.1
Osteoarthritis has all the hallmarks of a serious condition as defined by the FDA. It is associated with a range of levels of disability severity from mild, when it may cause intermittent pain with only minimal difficulty performing daily activities, to severe with chronic pain, progressive irreversible structural damage, and consequential progressive loss of function, often with associated decline in mental health as well as an increase in mortality when a person is no longer able to walk or live independently. Pain from arthritis is one of the key barriers to maintaining physical activity.
There are numerous non-pharmacologic and pharmacologic interventions for OA, and integrated models of patient-centered multi-disciplinary care have been shown to reduce pain and improve function and quality of life among individuals with OA, however there is no known cure or proven strategy for reducing progression from early to end-stage OA. There is no proven remedy for preventing the need for total joint replacement, which is the end result for millions of patients worldwide.2
While research continues for new OA therapies, the complex multi-tissue nature of the disease has made it difficult to target pathways for pharmacologic intervention. There are important regulatory considerations, specifically whether pursuing the pathway intended for a drug that treats a serious condition (i.e., accelerated pathway) can demonstrate an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. Currently, the ability to reliably predict treatment effects on common measures of structural progression and how a patient functions, feels, or survives has not been established.
1. https://oaaction.unc.edu/oa-module/oa-prevalence-and-burden/
2. Osteoarthritis: A Serious Disease. OARSI 2016.
We are thankful to the following Sponsors: